Discover how to create a clinical management plan with simple steps, examples, and expert tips. Designed for Canadian healthcare providers who want to improve team care, reduce errors, and stay compliant.
Introduction
Creating a Clinical Management Plan (CMP) is one of the most crucial responsibilities in modern healthcare—yet it’s often misunderstood, inconsistently applied, or insufficiently documented. Whether you’re a supplementary prescriber, a nurse practitioner, or part of a multidisciplinary care team, understanding how to develop and apply a CMP can enhance patient safety, improve coordination, and ensure legal compliance.
This guide is designed specifically for healthcare professionals in Canada, offering a step-by-step breakdown of how to create an effective clinical management plan. You’ll find actionable strategies, a sample template, regulatory insights, and practical tips that go beyond mere definitions.
What Is a Clinical Management Plan?
A clinical management plan is a formal, patient-specific document that outlines an agreed course of treatment between an independent prescriber and a supplementary prescriber. It includes key details such as medication protocols, treatment parameters, responsibilities, monitoring schedules, and review dates.
It is a vital component in supplementary prescribing, ensuring that every prescriber works within a defined scope based on collaborative planning. These plans are typically required in settings where non-medical prescribers operate under the authority of an independent physician, especially in chronic disease management and hospital discharge scenarios.
Clinical Management Plan vs Care Plan: Key Differences
| Feature | Clinical Management Plan | General Care Plan |
|---|---|---|
| Purpose | Focused on prescribing & treatment decisions | Broad overview of all patient care needs |
| Who Creates It | Independent + Supplementary Prescriber | Care coordinators, nurses, social workers |
| Legally Binding | Yes, must comply with prescribing regulations | Often not legally binding |
| Target Use Case | Medication & treatment under prescribing law | Holistic care planning |
While care plans offer a wide-angle view of patient support, clinical management plans zero in on prescribing responsibilities—ensuring there is no ambiguity in who is doing what, when, and why.
Why It Matters: Legal, Clinical, and Collaborative Benefits
The importance of clinical management planning cannot be overstated. These plans do more than outline treatments; they define legal prescribing boundaries, support multidisciplinary collaboration, and align care with clinical governance standards.
A well-documented CMP helps in:
- Preventing medication errors through clear prescribing responsibilities.
- Ensuring regulatory compliance with frameworks like Health Canada’s guidelines.
- Enhancing accountability in collaborative care teams.
- Providing a legal safeguard in cases of treatment disputes or adverse outcomes.
- Improving patient outcomes through structured review schedules.
In the UK and increasingly in Canada, organizations like The National Institute for Health and Care Excellence (NICE) offer structured recommendations for CMP development. These protocols help healthcare providers implement standardized processes while allowing for individualized patient care.
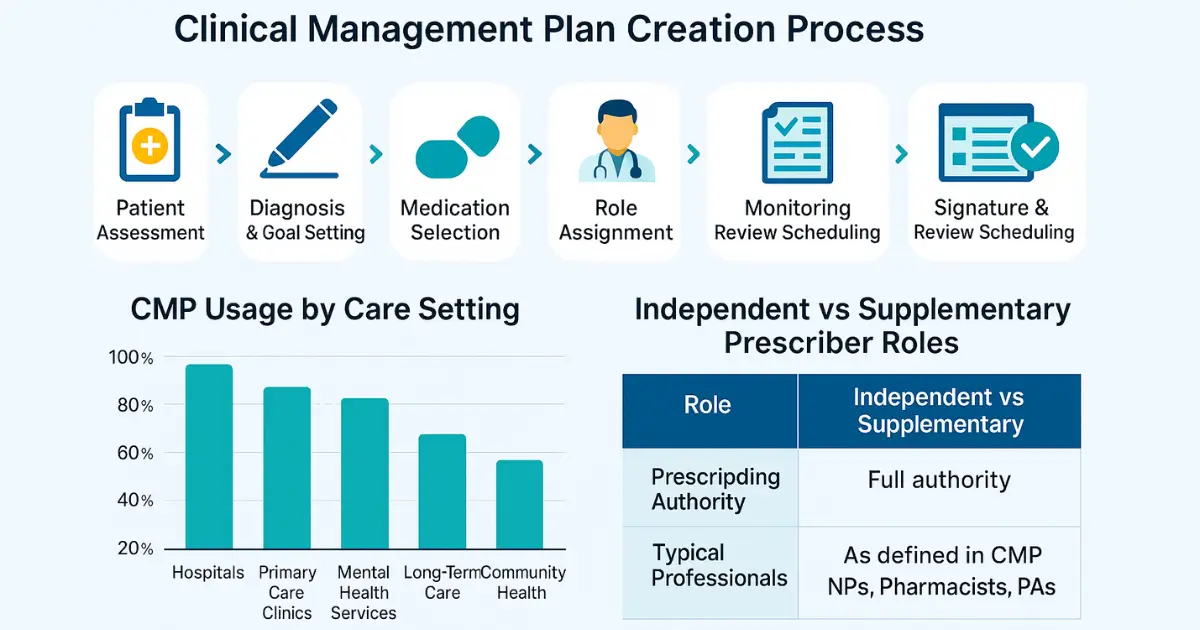
Who Creates & Uses a CMP?
A clinical management plan is typically developed by:
- An independent prescriber: Usually a physician or consultant who initiates the diagnosis and overarching treatment plan.
- A supplementary prescriber: This can include nurse practitioners, pharmacists, or other regulated health professionals with the authority to prescribe under CMP guidance.
In practice, the CMP becomes a shared reference point for:
- Physicians
- Nurse practitioners
- Clinical pharmacists
- Physiotherapists
- Respiratory therapists
- Case managers
Each professional uses the CMP to understand their role, document interventions, and align treatment delivery with the broader team’s goals.
Managed Clinical Networks Explained: How They Improve Healthcare Access, Quality & Coordination
When & Where to Use a Clinical Management Plan
A clinical management plan is most effective when patient care involves shared prescribing responsibilities between a medical doctor and another qualified healthcare provider. These plans are most commonly applied in:
- Chronic disease management (e.g., diabetes, hypertension, COPD)
- Post-discharge treatment plans
- Palliative and supportive care
- Rehabilitation programs
- Community mental health services
- Long-term medication regimens in outpatient settings
The CMP becomes a crucial operational document in any environment where ongoing pharmacological treatment needs close monitoring and delegation across healthcare professionals.

Common Healthcare Settings Using CMPs
| Setting | Typical Use of CMP |
|---|---|
| Hospitals | Discharge planning, post-operative medication |
| Primary Care Clinics | Chronic disease follow-ups, long-term prescriptions |
| Mental Health Services | Antipsychotic prescribing and patient monitoring |
| Long-Term Care Facilities | Multi-drug management for elderly patients |
| Community Nursing Programs | Shared medication oversight for home-care patients |
In Canada, CMPs are particularly encouraged under collaborative healthcare models such as Ontario Health Teams (OHTs) and Interdisciplinary Primary Care Networks. These models advocate integrated patient management with multiple care providers sharing responsibilities, which makes a well-defined clinical management plan essential for role clarity and patient safety.
In some cases, CMPs are mandatory for regulatory approval of non-medical prescribing. For example, the Canadian Nurses Association outlines standards for nurse practitioners which emphasize formalized treatment plans, especially when multiple prescribers are involved.
Step-by-Step: How to Develop a Clinical Management Plan
Creating a clinical management plan involves more than documentation. It is a structured process that ensures medical and legal clarity while accommodating patient-specific needs.
Step 1: Assess the Patient
Begin with a comprehensive assessment including diagnosis, co-morbidities, current medications, and psychosocial factors. The independent prescriber initiates this evaluation, determining whether a CMP is appropriate.
Step 2: Define Treatment Goals
Set clear, measurable goals. For instance:
- “Achieve HbA1c <7% within 3 months”
- “Stabilize blood pressure below 140/90 mmHg”
Goals must be clinically justified and documented in the plan.
Step 3: Outline Prescribing Responsibilities
Clarify exactly who is allowed to prescribe what, including dosage ranges, adjustment authority, and any exclusions.
| Medication | Prescriber Type | Dosing Limits | Review Frequency |
|---|---|---|---|
| Metformin | Supplementary Prescriber | Up to 2000mg/day | Every 3 months |
| Ramipril | Independent Prescriber | All dosing decisions | Bi-annual |
| Lorazepam (PRN) | Supplementary Prescriber | Up to 2mg per dose | Weekly |
This avoids overlap and legal liability issues, especially in complex or high-risk pharmacologic therapies.
Step 4: Establish Monitoring & Review Protocol
Specify how and when patient progress will be reviewed, who will conduct those reviews, and what clinical indicators will be monitored.
For example:
- Weight monitoring every 2 weeks
- Blood pressure logs maintained daily
- Monthly medication reconciliation
Step 5: Obtain Approval and Signatures
The plan must be signed by both the independent and supplementary prescribers, and ideally acknowledged by the patient or their legal representative. Store the signed copy securely within the patient’s medical record.
Step 6: Implementation & Record-Keeping
After implementation, any deviation from the CMP should be documented with justification. Plans should be version-controlled and retained according to institutional policy.
Step 7: Schedule for Review & Update
CMPs are living documents. Set a defined review period—typically every 3 to 6 months—or earlier if there’s a significant change in patient condition or treatment plan.
Clinical Supplies Management: A Simple Guide to Streamline Your Clinical Trials in 2025
Clinical Management Plan Template & Walkthrough
To make the development process easier, healthcare providers often use a standardized clinical management plan template. While formats may vary by institution or region, most effective CMPs include the following core sections:
Essential Components of a CMP
| Section | Description |
|---|---|
| Patient Details | Name, ID, DOB, diagnosis, known allergies |
| Independent Prescriber Info | Name, registration number, contact info |
| Supplementary Prescriber Info | Credentials, prescribing authority, contact info |
| Clinical Conditions Covered | All diagnoses relevant to the treatment plan |
| Medications & Parameters | Name, dose, route, frequency, permissible ranges |
| Monitoring Requirements | Frequency, parameters to monitor (e.g., BP, INR, blood glucose) |
| Review Schedule | Timelines for evaluation and renewal of the plan |
| Signatures & Dates | Legal authentication from involved prescribers and optional patient sign-off |
Using a well-structured template not only supports compliance with regulatory requirements but also ensures consistency across providers and facilities. Healthcare institutions in British Columbia and Alberta have already incorporated CMPs into their interdisciplinary electronic health record systems, streamlining care across teams.
Example Excerpt: Type 2 Diabetes CMP
Below is a simplified excerpt of a CMP tailored for a patient managing type 2 diabetes with the support of a nurse practitioner:
| Field | Details |
|---|---|
| Diagnosis | Type 2 Diabetes Mellitus |
| Prescribing Authority | NP Jane Doe, Nurse Practitioner, CRNP #56789 |
| Medication Plan | Metformin 500mg BID, titrate up to 1000mg BID as tolerated |
| Monitoring | Fasting glucose weekly; HbA1c every 3 months |
| Review Date | August 1, 2025 |
| Notes | Patient educated on hypoglycemia signs; advised on lifestyle changes |
Templates like this can be embedded into EMRs or used as printable forms, depending on institutional workflow.
You can refer to the British Columbia College of Nurses and Midwives for detailed prescribing frameworks relevant to CMP creation and implementation.
Legal and Regulatory Considerations in Canada
In Canada, the development and use of a clinical management plan are governed by both provincial regulations and professional standards set by licensing bodies. Each province outlines specific conditions under which supplementary prescribing is allowed, and in most cases, a signed CMP is a prerequisite for legal prescribing by nurse practitioners, pharmacists, or physician assistants.
Key Legal Requirements by Region
| Province | Regulatory Body | CMP Mandate |
|---|---|---|
| Ontario | College of Nurses of Ontario | CMP required for collaborative prescribing |
| British Columbia | BC College of Nurses and Midwives | Detailed prescribing standard with documented plan |
| Alberta | College of Registered Nurses of Alberta | Requires documented care agreement for shared prescribing |
| Nova Scotia | Nova Scotia College of Nursing | CMP encouraged for interdisciplinary teams |
For regulated professionals, failure to implement or follow a documented clinical management plan can lead to disciplinary action, especially if it results in patient harm or deviation from established scopes of practice.
Organizations such as the Canadian Medical Protective Association (CMPA) also stress the importance of team-based medication planning, highlighting that clear documentation of prescribing responsibilities reduces risk for both practitioners and patients.
Common Mistakes in Clinical Management Plans and How to Avoid Them
Despite the clear guidelines and legal frameworks, clinical management plans are often compromised by common errors that can reduce their effectiveness or even lead to clinical risk. Understanding these pitfalls—and how to avoid them—is essential for healthcare professionals involved in team-based prescribing.
1. Vague or Incomplete Prescribing Instructions
One of the most frequent issues is ambiguity in prescribing permissions, such as failing to state dose titration limits or monitoring criteria. This can lead to under-treatment, overprescribing, or legal ambiguity regarding scope.
Solution: Every CMP should clearly define what each prescriber can and cannot do, using quantifiable parameters.
2. Failure to Update the CMP After Clinical Changes
Patients’ conditions often change, but their CMPs remain static. This results in outdated treatment plans that don’t reflect new diagnoses, medications, or response to therapy.
Solution: Include a mandatory review schedule (e.g., every 3 or 6 months) and document any interim updates following major clinical decisions.
3. Lack of Patient Involvement
Involving the patient in decision-making is a cornerstone of patient-centered care. However, many CMPs are developed without formal patient input, leading to confusion or non-adherence.
Solution: Educate the patient, obtain acknowledgment (preferably a signature), and ensure they understand the shared plan.
4. Poor Documentation or Storage
Clinical management plans that are not readily accessible to all team members (e.g., stored in a single paper file or siloed in one department) undermine collaborative care.
Solution: Upload CMPs into shared electronic health record (EHR) systems accessible to all authorized clinicians.
5. Ignoring Regional Regulatory Differences
Practitioners often assume that one CMP format is valid across all provinces. In reality, regulatory expectations differ significantly by region.
Solution: Refer to local guidance such as the College of Physicians and Surgeons of Ontario (CPSO) prescribing policy, which outlines documentation standards including the role of supplementary prescribers and shared accountability.
Summary of Common Mistakes & Remedies
| Mistake | Impact | How to Avoid |
|---|---|---|
| Vague prescribing roles | Medication errors, legal risk | Define scope clearly in CMP |
| Static or outdated plan | Inaccurate treatment, poor outcomes | Schedule regular reviews |
| Exclusion of patient input | Non-adherence, dissatisfaction | Involve patient during planning |
| Isolated documentation | Miscommunication, duplicate efforts | Use shared EHR platforms |
| Not aligning with local rules | Regulatory non-compliance | Follow provincial regulatory body guidelines |
A well-executed clinical management plan is not just a formality—it’s an active document that should evolve with the patient and be recognized as part of a broader clinical governance framework.
For additional risk mitigation strategies in collaborative prescribing, healthcare teams may benefit from reviewing standards from the Canadian Patient Safety Institute, which emphasizes interprofessional communication and accountability.
Questions on Clinical Management Plan
Understanding the practical aspects of a clinical management plan is critical for safe and effective implementation. Here are answers to some of the most commonly asked questions by healthcare professionals in Canada.
1. Who can initiate a clinical management plan?
A clinical management plan is typically initiated by an independent prescriber, such as a licensed physician or specialist. The supplementary prescriber—such as a nurse practitioner, pharmacist, or physiotherapist—can then contribute to and operate within the plan, provided all responsibilities are clearly outlined.
In jurisdictions like Ontario and Alberta, the inclusion of regulated professionals in prescribing roles is strictly governed by bodies such as the Ontario College of Pharmacists and College of Nurses of Alberta.
2. Is patient consent mandatory for a CMP?
While formal written consent may not be universally required by regulation, it is considered best practice to involve the patient actively in the development of their treatment plan. This helps ensure transparency, build trust, and support adherence to the therapeutic regimen.
Consent may also be legally required depending on the setting—particularly in mental health or long-term care environments.
3. How often should a CMP be reviewed?
CMPs should be reviewed:
- At minimum every 3 to 6 months
- Immediately after a significant change in the patient’s health status or medication plan
- Following hospital discharge, particularly if new treatments or diagnoses have been introduced
4. Can a clinical management plan be used across care settings?
Yes, a single CMP can span multiple care settings—such as hospital, community, and home care—provided all involved providers have access to the most current version and the patient’s context remains relevant. However, regional interoperability remains a challenge in some provinces where EHR systems are not yet unified.
Regulatory FAQs by Province
| Province | Review Frequency Suggested | Patient Signature Required? | EHR Integration Status |
|---|---|---|---|
| Ontario | Every 3–6 months | Recommended, not required | Partial (via OntarioMD) |
| British Columbia | Every 6 months | Often required by employer | Strong integration (CareConnect) |
| Alberta | Upon clinical change | Encouraged | Integrated (Netcare) |
| Nova Scotia | Every 3 months | Optional | Developing |
These standards reflect growing expectations for collaborative prescribing models and the role of CMPs as a living record of shared treatment responsibility.
5. What should I do if roles are unclear in an existing CMP?
Unclear responsibilities create legal and clinical risks. If you find ambiguity in a current clinical management plan, it’s essential to:
- Review it with the team
- Clarify each prescriber’s scope of practice
- Revise and re-sign the document as needed
This process should be formally documented and stored, either digitally or in the patient’s medical file.
For guidance, refer to structured prescribing frameworks available through Health Quality Ontario.
Conclusion: Building Stronger Care Through Better Clinical Management Plans
A clinical management plan is not just a regulatory requirement—it is a foundational tool for delivering safe, coordinated, and patient-centred care in Canada’s evolving healthcare landscape. As team-based models expand and prescribing authority is shared across professionals, CMPs ensure everyone involved is aligned with the patient’s treatment goals and clinical context.
Well-developed plans reduce medication errors, clarify prescriber responsibilities, and establish legal safeguards for all parties. Moreover, they enhance collaboration across disciplines—something that’s increasingly vital in high-pressure environments like primary care networks, long-term care, and post-acute recovery.
Clinicians who invest time in creating effective CMPs not only improve patient outcomes but also ensure they are operating within provincial legal frameworks and best practices outlined by health authorities like Canada Health Infoway.
Key Takeaways
| Key Element | Summary |
|---|---|
| Definition | A shared treatment and prescribing plan between healthcare professionals |
| Who Uses It | Independent and supplementary prescribers including NPs, pharmacists |
| Legal Value | Legally binds prescribers to clearly defined roles and review schedules |
| Best Practice | Regular review, patient involvement, shared EHR documentation |
| Common Mistakes to Avoid | Ambiguity in scope, outdated plans, lack of patient engagement |
| Regional Differences | Regulations and documentation standards vary by province |
By integrating CMPs into daily clinical workflows and digital systems, Canadian healthcare professionals can meet both regulatory expectations and the growing demand for integrated care.
FAQ
What is a clinical management plan?
A clinical management plan is a written agreement between healthcare providers that outlines a patient’s treatment and prescribing responsibilities.
Who can write a clinical management plan?
Usually, an independent prescriber like a doctor starts the plan, and a supplementary prescriber such as a nurse or pharmacist can contribute to it.
Is a clinical management plan legally required in Canada?
It depends on the province. Some provinces require it for shared prescribing. It is always a best practice to use one.
How often should a clinical management plan be reviewed?
A plan should be reviewed every 3–6 months or whenever the patient’s condition changes significantly.
Does a patient need to sign the clinical management plan?
While not always required, getting the patient’s signature shows involvement and can help with treatment compliance.
Can a CMP be used in hospitals and community care?
Yes, a clinical management plan can be used across multiple care settings as long as it stays updated and accessible to all team members.
What should be included in a clinical management plan?
It should include patient details, medication plans, roles of each prescriber, review timelines, and monitoring instructions.


About Author
Related Posts
Women’s Health Clinics in Canada: Comprehensive Care for Every Life Stage
Managed Clinical Networks Explained: How They Improve Healthcare Access, Quality & Coordination
Canada's Doctor Shortage: Causes, Impacts, and Solutions for a Healthier Future
How to Find the Right Gynecologist in Canada: A Step-by-Step Women’s Health Guide